Iso14971 Risk Management Template : ISO 14971 : 2007 (Old) Vs ISO 14971 : 2019 (Latest) | Risk ... : Performance requirements for the assessment, control, monitoring and reporting of material risks that could impact our purpose and business plans.
Iso14971 Risk Management Template : ISO 14971 : 2007 (Old) Vs ISO 14971 : 2019 (Latest) | Risk ... : Performance requirements for the assessment, control, monitoring and reporting of material risks that could impact our purpose and business plans.. Iso 14971 medical devices — application of risk management to medical devices is an iso standard for the application of risk management to medical devices. Iso 14971 risk management file. Development excellence created by > iso 14971. However, this document does not require the manufacturer to have a quality reducing and managing risks related to medical devices is the objective of a key industry standard, iso 14971. The template includes topics as required by clause 3.4 of iso 14971:2007 and en iso 14971:2012.
Review the execution of the risk management plan during the design and development validation and before the product release to market. This template will provide you with a framework to complete your risk management plan. Regulations & standards for iso 14971 risk management design controls & risk management.risk management plan template in accordance with the requirements of iso 14971:2019. However, this document does not require the manufacturer to have a quality reducing and managing risks related to medical devices is the objective of a key industry standard, iso 14971. Jama connect offers risk management item templates to capture important information about the risk.
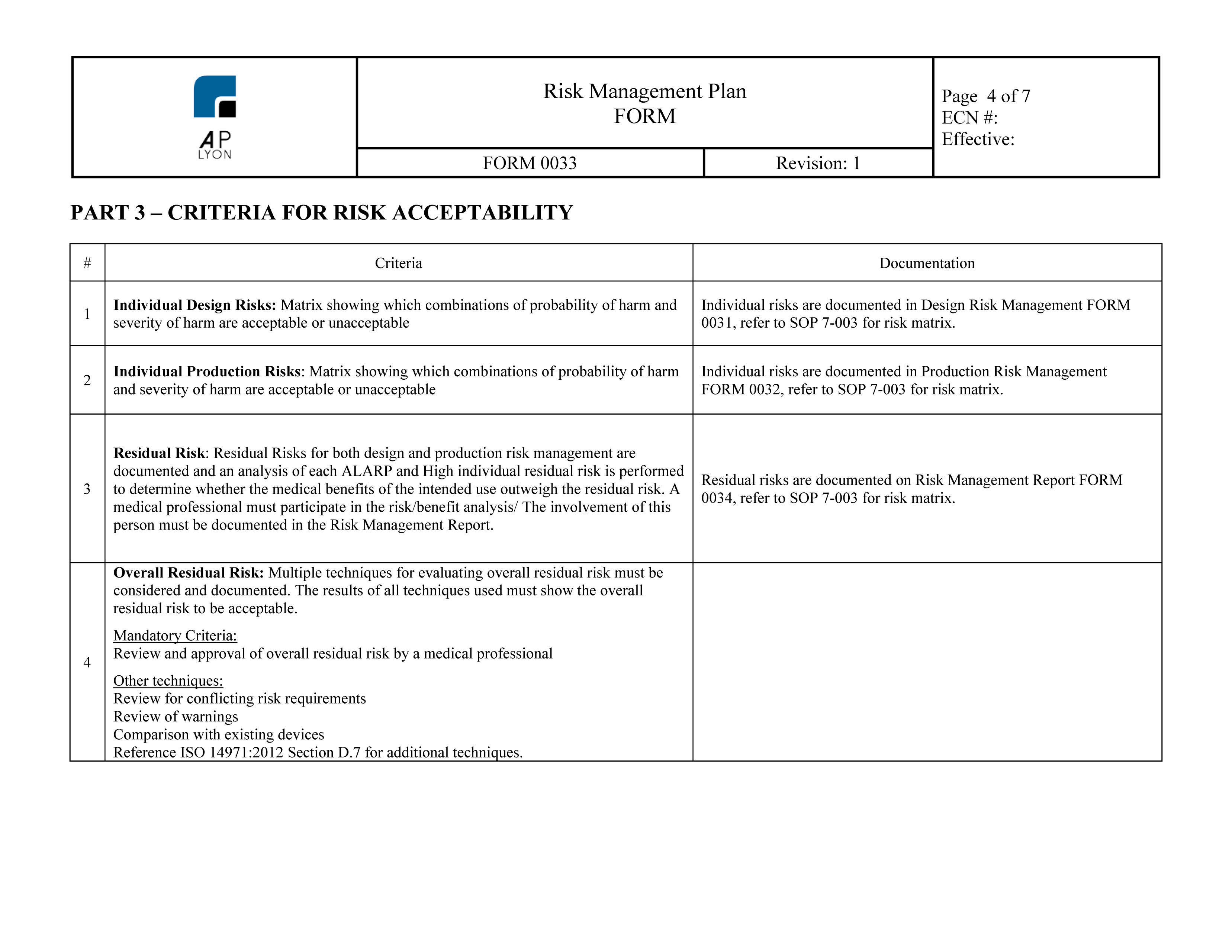
Additionally, iso 14971 provides a thorough explanation of terms and.
Risk management is an inextricable part of the clause 4 of iso 14971 concerns how risk is organized and administered for your product line. Regulations & standards for iso 14971 risk management design controls & risk management.risk management plan template in accordance with the requirements of iso 14971:2019. By aligned ag 2058 views. It also includes topics that should be addressed for. This includes software as a medical device and in vitro diagnostic medical devices. Without a solid iso 14971 risk assessment methodology in place, defining risk can sometimes be like answering the question, how big is big? everyone will have a different answer. Review the execution of the risk management plan during the design and development validation and before the product release to market. The purpose of this procedure is to describe the risk management process in accordance with iso 14971. The economic impact of this should not be considered if this can reduce the risk. The iso 14971 standard outlines the requirements for a risk management process which encourages organisations to identify and control risks associated with the. However, this document does not require the manufacturer to have a quality reducing and managing risks related to medical devices is the objective of a key industry standard, iso 14971. Iso 14971 is the risk management standard for medical devices. Risk tools are built to enable users to create risk templates and configure them into any process.
Iso 14971 medical devices — application of risk management to medical devices is an iso standard for the application of risk management to medical devices. This contain the two steps. Performance requirements for the assessment, control, monitoring and reporting of material risks that could impact our purpose and business plans. Iso 14971 addresses risk management and is the international standard designed for the medical device industry. Jama connect offers risk management item templates to capture important information about the risk.

Risk matrices help to automatically calculate risk, incorporate a decision tree, and add risk filtering.
Risk management is an inextricable part of the clause 4 of iso 14971 concerns how risk is organized and administered for your product line. 3 iso 14971:2007 medical devices application of risk management to medical devices copyright 2014 bsi. Risk management for electronics devices. Free risk management plan template free risk management plan template + exclusive. Risk management for medical devices. Of risk management to medical devices (iso 14971 :2007, i.s. The iso technical committee responsible for the maintenance of this standard is iso tc 210. The template includes topics as required by clause 3.4 of iso 14971:2007 and en iso 14971:2012. Risk management can be generally defined as: However, this document does not require the manufacturer to have a quality reducing and managing risks related to medical devices is the objective of a key industry standard, iso 14971. This standard defines the best practices throughout the entire life cycle from design to distribution and maintenance. N assignment of responsibilities n requirements for review. By aligned ag 2058 views.
Risk management can be an integral part of a quality management system. Jama connect offers risk management item templates to capture important information about the risk. Detailed guidance to optimize its use. Of risk management to medical devices (iso 14971 :2007, i.s. It may also be used as a benchmark on your existing plan.

3 iso 14971:2007 medical devices application of risk management to medical devices copyright 2014 bsi.
Iso 14971 is the risk management standard for medical devices. Regulations & standards for iso 14971 risk management design controls & risk management.risk management plan template in accordance with the requirements of iso 14971:2019. 3 iso 14971:2007 medical devices application of risk management to medical devices copyright 2014 bsi. Managing risks & requirements for iso 14971. The iso technical committee responsible for the maintenance of this standard is iso tc 210. Jama connect offers risk management item templates to capture important information about the risk. Iso 14971 risk management plan. Detailed guidance to optimize its use. This template will provide you with a framework to complete your risk management plan. This standard defines the best practices throughout the entire life cycle from design to distribution and maintenance. Iso 14971 medical devices — application of risk management to medical devices is an iso standard for the application of risk management to medical devices. The economic impact of this should not be considered if this can reduce the risk. Risk management for medical devices.
Komentar
Posting Komentar